Healthcare, Life Sciences, & Bio-Technology
Software Testing, Development,
and Validation Tools
When developing medical devices or healthcare information systems, accuracy and precision are paramount. You need to make sure that the testing and validation procedures meet the requirements of the FDA / EMA, and specifically 21 CFR Part 11. Comprehensive software development practices and extensive testing ensures that your healthcare, biotech, or medical software functions flawlessly. Inflectra provides the capability for managing your testing and compliance activities to meet these strict requirements. We understand your world, and our software solutions help you develop medical devices, validate patient systems, perform healthcare app testing, evaluate wearable device performance, and much more.
Compliance Challenges
Whether developing or testing medical devices, managing patient data, or coordinating care, compliance is critical. Life sciences are heavily regulated, and the regulations are constantly changing. You need to perform continuous testing and validation, with real-time traceability.
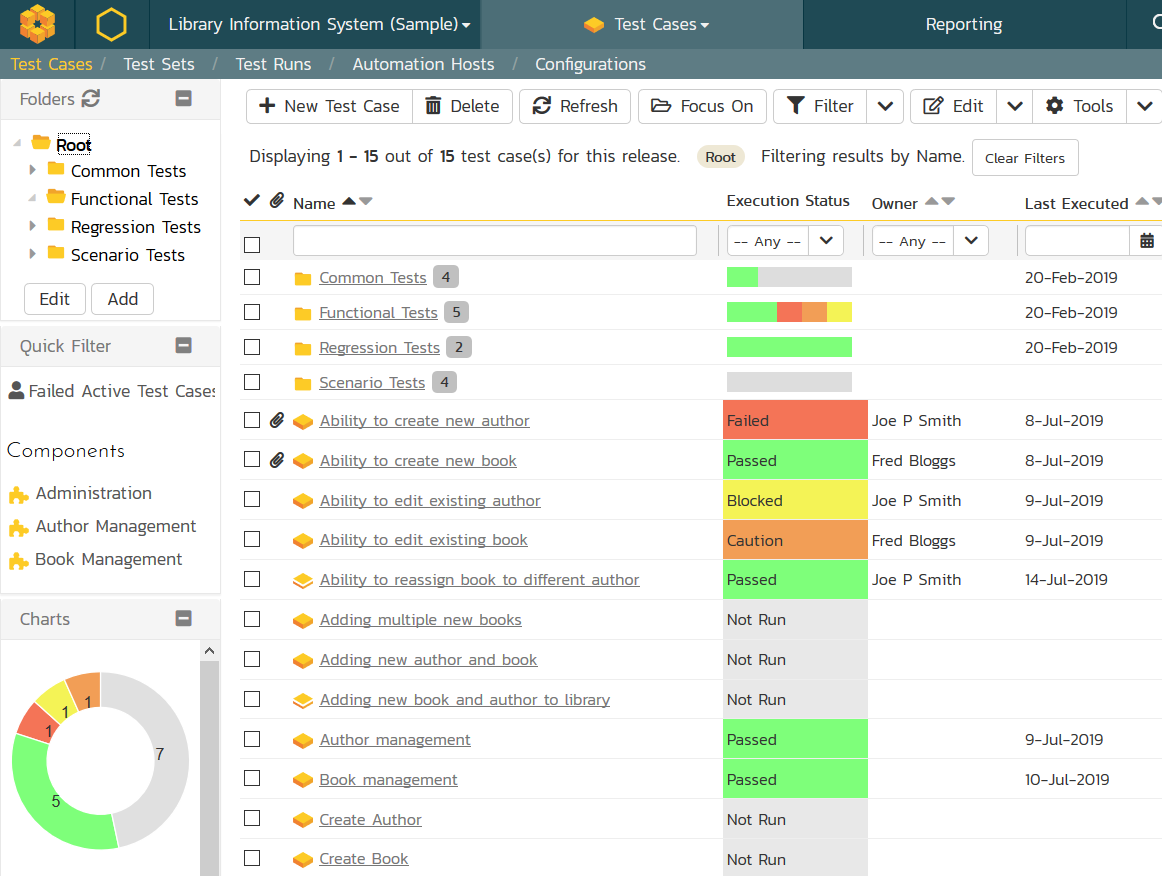
Tools You Can Trust
Inflectra provides the capability for managing your testing and validation activities to meet these requirements. We have proven turnkey solutions that give you powerful requirements traceability and test management, with powerful reporting and workflows that streamline compliance.
"Elevating Pharmaceutical Validation"
TCell ws looking to streamline the creation and management of pharmaceutical industry validation documents. As Jira fell short in addressing the unique demands of pharmaceutical validation, TCell migrated to SpiraTeam by Inflectra, that included the essential features for the pharmaceutical sector out-of-the-box. Inflectra provided TCell with a competitive edge, turning regulatory compliance from a challenge into a strategic asset. Pharmaceutical validation documents were created in record time with Inflectra, demonstrating our unwavering focus on quality.Ram S., Managing Director, TCell Clinical Services
Software Solutions For You, Whatever Your Focus
Healthcare
Our tools help healthcare providers and payers manage complex and shifting regulatory and attestation needs - from HIPAA compliance and electronic medical records, to financial traceability and reconciliation.
Bio-Technology
Move beyond paper-based testing and validation with Inflectra. Whether developing new pharmaceuticals or medical devices, you need FDA-compliant 21 CFR Part 11 traceability and compliance.
Life Sciences
SpiraTest and SpiraTeam give you accurate information and real-time reporting. We understand how critical this is for the safety of the public, whether you are running clinical trials or managing public health programs.
Features for Healthcare & Life Sciences
Validation & Testing
Replace your paper-based methods with streamlined workflows and testing using our integrated validation solution.
Electronic Signatures
Our products include a unique, built-in 21 CFR Part 11 electronic signature facility that is tightly integrated with our customizable workflows to ensure compliance.
Data Privacy
Keep all your project artifacts in a secure, private, HIPAA-compliant repository. Whether you choose cloud hosting, or prefer to install on-premise, we have you covered.
End-to-End Traceability
Use our platform to define your specifications, create test plans and test scenarios, execute manual or automated testing, and tie corrective actions to the results, with real-time reporting.
Audit Trails
Our platform automatically maintains a secure audit log of all actions in the system, with the user, timestamp, changes and reason all captured and stored.
Automated Validation
Save time validating your systems. Our platform includes automation tools that generate test scenarios and scripts directly from your running applications.
Trusted By Industry Leaders




Featured Resources
Take A Deeper Dive
Whitepapers & Articles
- We just did our FDA Audit. It was scheduled for 5 hours. With Spira It Took 90 Minutes!
- AI in Healthcare: How It's Used, Benefits, & More
- FDA Validation & Testing with 21 CFR Part 11
- SpiraTeam HIPAA Compliance Checklist
- Enhancing ICH E6(R3) Compliance with Inflectra's SpiraPlan and Rapise
- Project Management In The Drug Development Supply Chain
- Preparing for the GAMP Transition to Computer Software Assurance
- Configuring SpiraTeam for Testing in Validated Environments
- Importance of Testing Software for Life Sciences
- Medical Device Testing: Different Types & Trends
Try Inflectra For Free Today
Easy To Migrate
It's incredibly easy and user-friendly to switch to our platform. You can migrate your legacy data, documents, spreadsheets, and other critical information from other tools using our out-of-the-box migration features.
Free Trials
We encourage you to try our products in your environment. That's why we offer a 30-day free trial (no credit card needed), with all the functionality and our legendary technical support included.
Exceptional Service
Our products come with industry best practices baked in. They have over 70 out of the box integrations, default workflows and industry templates. All products include unlimited world-class technical support.